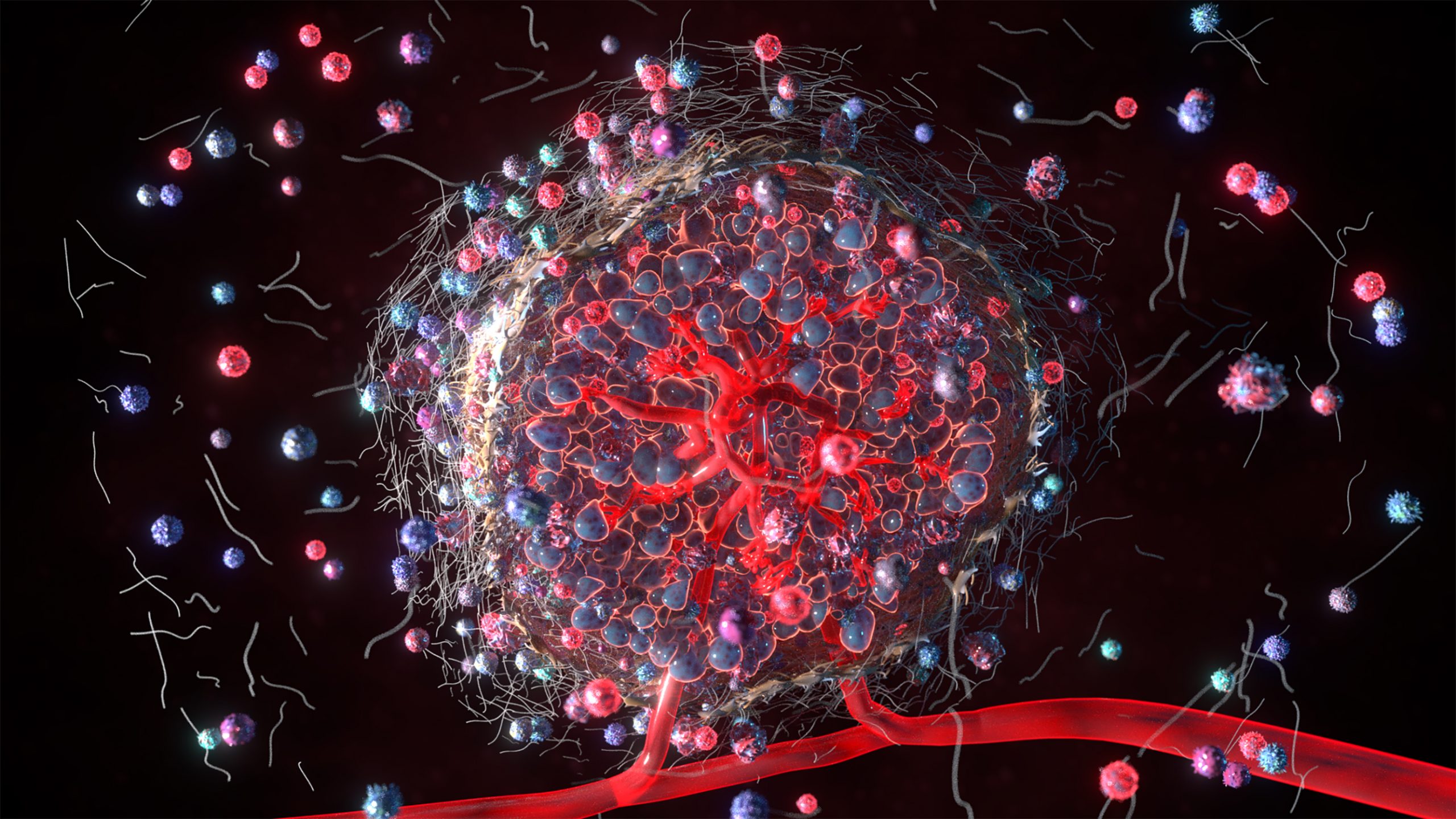

The Training Centre for Personalised Therapeutics Technologies is a important research centre.Our Centre aims to advance and deploy new technologies that will remove long-standing barriers to new drug discovery and development, and excitingly, provide opportunities for highly effective personalised treatments. Our Centre, through training a new generation of biomedical researchers with the appropriate entrepreneurial/commercial skills, alongside technology development, will enable us to transform the way the Medtech/Pharma industry accesses emergent technologies and translate research discoveries into benefits for end-users sooner and more effectively. The Centre will foster a vibrant, sustainable, growing industry of highly skilled researchers using organ-on-a-chip and bioprinting technologies that will support improved selection of therapeutics for investment in clinical trials.Pharmacology and Therapeutics and the Department of Biochemistry and Molecular Biology brings benefits to both units in terms of critical mass, interdisciplinary teaching and the development of an integrated research program spanning discovery science trough to drug development. Both departments have long and proud histories, and have now come together to produce a department with a remarkable breadth and depth in research expertise that underpin our key themes of molecular understanding of disease, translational research, drug discovery and development.
Biochemistry and Molecular Biology has research strengths in molecular cell biology, structural biology and macromolecular imaging, protein biochemistry, molecular immunology, neurobiology and computational biology. We apply our strengths to diseases such as cancer, neurodegenerative disorders, malaria, parasite infections and inflammatory autoimmune diseases.Research Fellow in Pharmacology and Therapeutics will be actively involved in research and teaching of pharmacology of the cardiovascular and central nervous system, respiratory pharmacology and molecular pharmacology.
Focus remains on information technology with development of multimedia teaching methods and there are strong links with the pharmaceutical industry thanks to our collaborative research programs.


About board-certified clinical research coordinators (CRC)
A CRC (Clinical Research Coordinator) is an essential professional engaged in the coordination and achievement of clinical research, and who supports doctors conducting clinical research, pharmaceutical industrial sponsors, participating volunteers, and also cooperates with various staff in medical institutions. It is necessary for all medical drugs, devices, and technology to undergo clinical research evaluating efficacy and safety before being used as medical treatments in clinical practice. Therefore, appropriate, qualified clinical trials must be conducted.
The Japanese Society of Clinical Pharmacology and Therapeutics established the accreditation system for the Board-Certified CRC in 2003. A Board-Certified CRC is identified as a CRC who contributes to the appropriate and smooth performance of clinical trials, providing the general public with the benefit of more effective and safe medical technology. Board-Certified CRCs are now aiming for the development of up-to-date medical technology and also playing important active parts in the field of clinical research and trials, including trials of investigational new drugs.
To apply for the Board-Certified CRC examination, please go to our society;s homepage,
